Disclaimer: This web page was produced as an assignment for an undergraduate course at Davidson College.
Inhibiting the cytoskeletal-regulating ROCK kinase reduces adhesions, restoring migratory potential in aged neural stem cells to promote neurogenesis in the aging brain.
While we’re aware of the declining regenerative potential with age, the intricacies behind this remain largely obscure, particularly regarding migratory pathways and how potential defects to these areas can impact regeneration. Similarly, epigenomic studies, which explore the interplay between behavior, environment, and gene function, have yet to identify age-dependent changes across different cell types within the neurogenic niche, leaving a wealth of information untapped. The chromatin landscape, consisting of DNA, RNA, and proteins, can be a vital source for addressing these uncertainties. Through identifying changes within the chromatin landscape of neural stem cell niches, it is possible to reverse age-related defects and prevent brain aging, which is what Yeo et al.,1 set out to do.
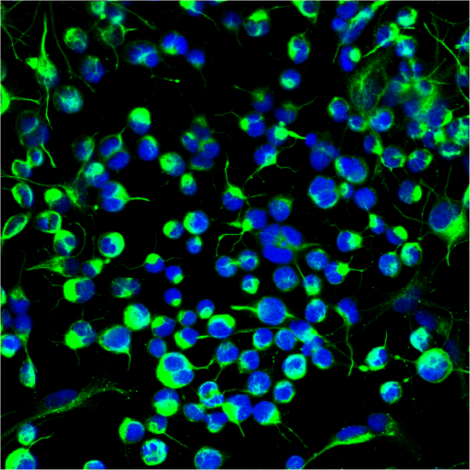
Researchers used both young and old mice to assess chromatin accessibility profiles across five distinct cell types within the subventricular zone (SVZ) neural stem cell niches. This region of the brain is known for its continuous production of new neurons and glia cells, making it an ideal region for studying regenerative capabilities.2 In this investigation, the mice expressed green fluorescent protein (GFP) under the promoter for glial fibrillary acidic protein (GFAP-GFP), which is a protein integral to the cytoskeletal structure of glial cells.3 Through using fluorescence-activated cell sorting (FACS), researchers were able to isolate five distinct cell types—endothelial cells, astrocytes, activated neural stem cells, neural progenitor cells, and quiescent neural stem cells—from young (3–5 months old) and old (20–24 months old) GFAP-GFP mice, allowing for a deeper analysis of age-related changes in chromatin accessibility. Endothelial cells prevent adhesion and help maintain smooth blood flow, astrocytes regulate synaptic function and aid in synaptic formation, activated cells promote regeneration of tissue and repair, neural progenitor cells are able to differentiate into various neuronal and glial cells, and quiescent neural stem cells play a crucial role in stem-cell renewal and regeneration.
Following this, an assay was performed to assess the quantity of transposase-accessible chromatin using sequencing (ATAC-seq). The results revealed significant chromatin accessibility enrichment around transcription start sites (TSSs). Further analyses, including Principal Component Analysis (PCA) and hierarchical clustering, effectively distinguished whether a cell was endothelial, quiescent, activated, or a progenitor. Researchers also examined the Ascl1 locus, which is a protein that regulates neural stem cell and glial transcription factors as well as chromatin modifying genes.4 They found greater chromatin accessibility in activated neural stem cells and progenitors compared to quiescent neural stem cells and astrocytes. These insights shed light on age-related chromatin alterations within distinct cell types from the SVZ neurogenic niche.
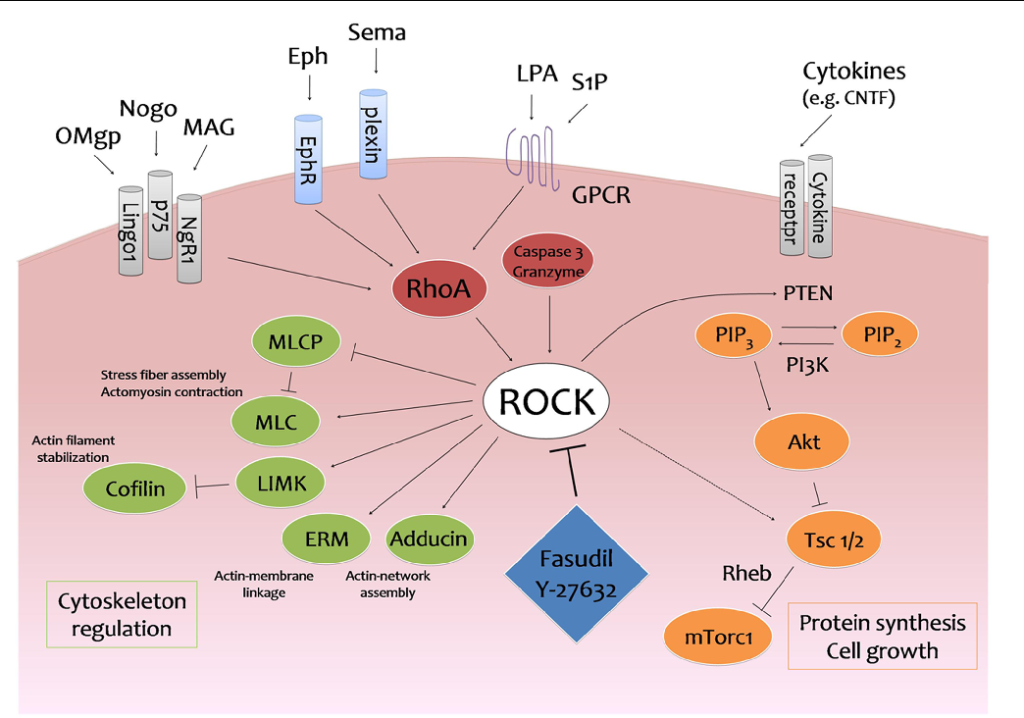
Yeo et al., also wanted to understand the heightened adhesion observed in activated NSCs (aNSCs) and progenitors during aging. They employed molecular tension sensors to assess cellular properties, particularly focusing on cell-matrix adhesion which is crucial for cell migration, tissue organization, and differentiation.5 The study revealed an age-related increase in average adhesion force and a higher proportion of active neural stem cells and neural progenitor cells exhibiting force-producing adhesions. Notably, the study highlighted the Gα12/13 signaling pathway’s role in regulating cell adhesion via the Rho-associated protein kinase (ROCK). In pharmological studies, it has been found that ROCK is involved in various diseases ranging from nerve injury to hypertension.6 Similarly, this kinase impacts the production of endothelial cells, which may explain increased adhesion with age. ROCK was found to be enriched in old activated neural stem cells and neural progenitor cells. By targeting ROCK with a small-molecule inhibitor, researchers reversed the age-related increase in adhesion strength, eliminating force-producing adhesions and focal adhesions in both young and old activated neural stem cells and neural progenitor cells.
With this knowledge, researchers wanted to understand ROCK inhibition on neural stem cell behavior and neurogenesis in both in vitro and in vivo settings. In vitro experiments revealed that ROCK inhibition improved the migration speed of old activated neural stem cells and reversed the age-related decline in cell dispersion. Furthermore, ROCK inhibition had a more significant impact on reducing focal adhesions in old activated neural stem cells and neural progenitor cells compared to young counterparts, without affecting cell differentiation, proliferation, or survival. In vivo experiments demonstrated that ROCK inhibition increased the distance of activated neural stem cells and neural progenitor cells from the ventricle in old mice and enhanced neurogenesis in the olfactory bulb, an area essential for smell. These findings further support that inhibiting the ROCK kinase can alleviate age-related deficits in neural stem cell behavior and promote neurogenesis in aging brains.
This study provides valuable insights into the chromatin dynamics of neural stem cells in aging animal models, but I believe that we should be somewhat careful in applying this to a human context. The controlled conditions of laboratory experiments with model organisms may not fully capture the complexities of cognitive aging in humans, where a multitude of factors such as environment, diet and health status, and general genetic variability play significant roles. Cognitive decline may vary across individuals and cultures. As I am interested in the intersection between Biology and Psychology, I have previously read up on a paper covering this topic, and it suggested that despite the cognitive decline that accompanies aging, numerous fundamental cognitive abilities and functions, particularly those deeply influenced by cultural factors, may not experience an equivalent level of functional deterioration. These skills and processes, often termed as “culturally saturated,” are developed through cultural acquisition. As a result, they possess the potential to counterbalance the decline in biological capabilities.7
References:
- Yeo, R.W., Zhou, O.Y., Zhong, B.L. et al. Chromatin accessibility dynamics of neurogenic niche cells reveal defects in neural stem cell adhesion and migration during aging. Nat Aging 3, 866–893 (2023). https://doi.org/10.1038/s43587-023-00449-3
- Alvarez-Buylla, A., Herrera, D. G., & Wichterle, H. The subventricular zone: source of neuronal precursors for brain repair. Progress in brain research 127, 1–11 (2000). https://doi.org/10.1016/s0079-6123(00)27002-7
- Yang, Z., & Wang, K. K. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends in neurosciences 38 (6), 364–374 (2015). https://doi.org/10.1016/j.tins.2015.04.003
- Vue, T. Y., Kollipara, R. K., Borromeo, M. D., Smith, T., Mashimo, T., Burns, D. K., Bachoo, R. M., & Johnson, J. E. ASCL1 regulates neurodevelopmental transcription factors and cell cycle genes in brain tumors of glioma mouse models. Glia, 68(12), 2613–2630 (2020). https://doi.org/10.1002/glia.23873
- Berrier, A. L., & Yamada, K. M. Cell-matrix adhesion. Journal of cellular physiology 213 (3), 565–573 (2007). https://doi.org/10.1002/jcp.21237
- Amano, M., Nakayama, M., & Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67 (9), 545–554 (2010). https://doi.org/10.1002/cm.20472
- Kitayama, S., The Aging Mind: Opportunities in Cognitive Research. Cultural Variations in Cognition: Implications for Aging Research (2000). https://www.ncbi.nlm.nih.gov/books/NBK44822/
- Tönges, L., Koch, J. C., Bähr, M., & Lingor, P. ROCKing Regeneration: Rho Kinase Inhibition as Molecular Target for Neurorestoration. Frontiers in molecular neuroscience, 4 (39), (2011). https://doi.org/10.3389/fnmol.2011.00039
For any questions related to this post, please reach out to Abraham Hernandez Mujica (abhernandezmujica@davidson.edu)
Skip back to main navigation
Abe, your study on how inhibiting the cytoskeletal-regulating ROCK kinase can promote neurogenesis in aging brains is really hopeful! As a companion caregiver for a patient with Alzheimer’s, I’m excited to see where this research leads. Your mention of the intersection between biology and psychology really connected with me, especially as I’ve seen how simple activities like going for walks or looking at old photos can improve my patient’s mood and memory. I am now more aware of how lifestyle choices can impact cognitive aging, and I plan to prioritize healthy eating, regular exercise, and social engagement to keep my brain healthy as I grow older! Thanks for sharing!
Abe, your study on how inhibiting the cytoskeletal-regulating ROCK kinase can promote neurogenesis in aging brains is really hopeful! As a companion caregiver for a patient with Alzheimer’s, I’m excited to see where this research leads. Your mention of the intersection between biology and psychology really connected with me, especially as I’ve seen how doing simple activities together like going for walks or looking at old photos can improve my patient’s mood and memory, likely by releasing neurotransmitters such as dopamine, serotonin, and acetylcholine. I am now more aware of how lifestyle choices can impact cognitive aging, and I plan to prioritize healthy eating, regular exercise, and social engagement to keep my brain healthy as I grow older! Thanks for sharing!