Microbiome competition experiments in mice have demonstrated that gut bacteria have the home court advantage when transplanted into the same species.
This web page was produced as an assignment for an undergraduate course at Davidson College.
The commensal microbiome is a profoundly important ecosystem of microorganisms that live in and on humans and other animals. Microbiotas are specifically adapted to their hosts, and disrupting the host-specific microbiome can negatively affect the host.1,2 Since the host immune system must learn to tolerate the microbiome, understanding the establishment of the microbiome remains a key area of research within immunology. However, much is still unknown about the evolutionary processes responsible for adaptations within the microbiota that confer specificity to the host.3 One possible explanation is that bacteria within the microbiome adapt to their specific hosts to increase their survival. In a recent study, Sprockett et al.4 set out to test this explanation.
To investigate whether microbiotas have an advantage in the host from which they are derived, Sprockett and colleagues performed a series of clever experiments that pitted native microbiotas against foreign. First, they created several mouse lines by collecting different species of wild mice (the house mouse species Mus musculus domesticus and the non-house mouse species Mus spicilegus, Mus pahari, and Peromyscus maniculatus) and breeding them in laboratory conditions for several generations. Next, they collected fecal samples from these mouse lines and identified the types of bacteria present so that they could monitor changes in microbiota composition. The researchers then mixed fecal samples from pairs of mice so that each mixture contained feces from a house mouse and a non-house mouse. These mixtures were then used to introduce a hybrid microbiota into a strain of lab mouse derived from the house mouse. The researchers monitored the composition of the microbiome for the next four weeks. Since the recipient mouse is a house mouse, a shift in microbiome composition towards the house mouse donor’s microbiome would indicate that the bacteria are adapted to house mice and have the home court advantage. Indeed, the researchers found that the native microbiotas from the house mouse consistently outcompeted the foreign microbiotas from the non-house mouse.
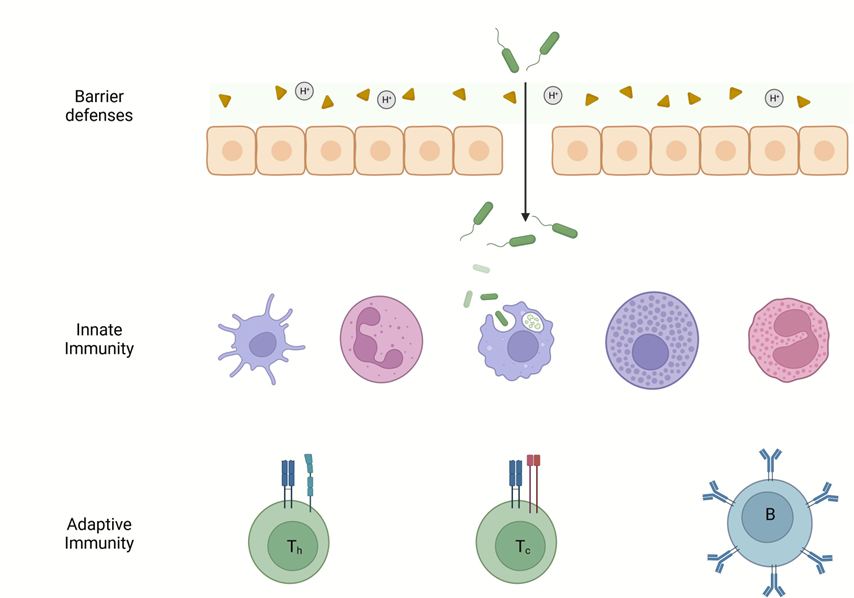
The three layers of defense. Created with BioRender.
Sprockett and his colleagues then wanted to know whether the mouse adaptive immune system was contributing to the home-court advantage enjoyed by native microbiota. Vertebrates have evolved three layers of defense against pathogens: a system of barrier defenses at the boundary between the organism and the outside world, an innate immune system that responds to broad categories of potentially dangerous microorganisms, and an adaptive immune system that provides powerful responses to specific pathogens (see illustration).5 The microbiota must adapt to all three layers of defense to successfully inhabit the host. To determine whether specific adaptations to the adaptive immune system were involved, the researchers used a special strain of lab mouse that does not have a functional adaptive immune system. Fecal samples from house mouse and non-house mouse strains were mixed as in the first experiment and introduced to both normal house mouse-derived lab mice and lab mice that lack an adaptive immune system. Interestingly, native microbiotas generally continued to outcompete microbiotas from non-house mice. However, a few of the native bacterial strains present were no longer favored in the mouse without adaptive immunity. These results suggest that most – but not all – bacteria with a home court advantage in the gut microbiome are not specifically adapted to the adaptive immune system.
Understanding how the gut microbiota is established is an important issue in immunology and in medicine. This study has provided considerable evidence to support the hypothesis that microbiotas have specific adaptations that enable them to inhabit their hosts. It is especially interesting that the adaptive immune system does not affect the performance of most bacterial species within the microbiota, as the adaptive immune system has many mechanisms of establishing tolerance and regulating immune responses. However, this study also has limitations. Notably, the competition experiments were only performed in a house mouse model. For completeness, a non-house mouse model is needed to conclusively demonstrate the home court advantage of native microbiotas.
As our understanding of the gut microbiome improves, we will likely target it with therapeutics. For instance, insults to the gut microbiome may increase susceptibility to infection with Clostridioides difficile, which can cause severe gastrointestinal symptoms.6 Supplementing these patients with healthy microbiota may be helpful in the treatment of this and similar diseases. Based on the results of this study, however, we may need to target different populations of patients with different microbial cultures to ensure optimal uptake of transplanted microbiotas. Overall, this study has made a necessary contribution to an area of research with potential relevance to both basic science and clinical relevance.
Article by Sheridan Page. Contact the author at shpage@davidson.edu
© Copyright 2024 Department of Biology, Davidson College, Davidson, NC 28036
References:
1. Zmora, N. et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 174, 1388-1405.e21 (2018). Article
2. Brooks, A. W., Kohl, K. D., Brucker, R. M., Opstal, E. J. van & Bordenstein, S. R. Phylosymbiosis: Relationships and Functional Effects of Microbial Communities across Host Evolutionary History. PLOS Biol. 14, e2000225 (2016). Article
3. Groussin, M., Mazel, F. & Alm, E. J. Co-evolution and Co-speciation of Host-Gut Bacteria Systems. Cell Host Microbe 28, 12–22 (2020). Article
4. Sprockett, D. D. et al. Home-site advantage for host species–specific gut microbiota. Sci. Adv. 9, eadf5499 (2023). Article
5. Paludan, S. R., Pradeu, T., Masters, S. L. & Mogensen, T. H. Constitutive immune mechanisms: mediators of host defence and immune regulation. Nat. Rev. Immunol. 21, 137–150 (2021). Article
6. Sehgal, K. & Khanna, S. Gut microbiome and Clostridioides difficile infection: a closer look at the microscopic interface. Ther. Adv. Gastroenterol. 14, 1756284821994736 (2021). Article
These findings are actually incredibly interesting; I had no idea the gut microbiome was influenced by the immune system. I am also curious about the evolutionary history of the preferred bacteria of the microbiome: are there similar microbiomes across species/within species; do the gut bacteria evolve alongside species; how is the microbiome established; etc. I also wonder how these findings might differ outside of the mice species tested, which I think is a reasonable next step to pursue in this research, as you said. Overall, this is very well written; it is concise and layman friendly.
It is fascinating how diverse the gut microbiome is in species and between people that the gut microbiome would not have been understood without the technological advancements in genomics over the past couple of decades. I love how the article highlights the importance of the immune system response to potential pathogens and how the bacterial microbes can either adapt to new environments or are outcompeted by native-favored microbiota. As the gut lining is one of the primary tissues where nutrients and minerals are absorbed, this location also may allow for the absorption for malicious pathogens to cross over at the first opportunity. The intersection between immunity and the gut microbiome composition is especially important for patients susceptible to certain bacteria types, and further studies of microbiome variance in humans can eventually provide novel therapies in gut microbiome-related illnesses.
Fascinating article! The visual representation of the immune system was really helpful in understanding the layers.
I would be interested in seeing this experiment done with irradiated mice (mice who have been subjected to radiation to destroy the immune cells) that have received a different mouse’s immune cells as a replacement immune system. In this experiment, if the host immune system really does have a home court advantage, we would expect to see the microbiome of the donor mouse (whose cells were put into the irradiated mouse) out-compete the irradiated mouse’s original microbiome. This would help solidify that the host microbiome had an advantage.
It would also be interesting to see what types of native bacteria were no longer favored. It’s possible they belong to the same group, like Gram (+) or Gram (-).